Exploring Ionic And Covalent Bonds Gizmo : Ionic Bonds Gizmo Worksheet Answers - worksheet : The main difference between ionic bonds and covalent bonds is sharing of electron pairs and atoms.
Exploring Ionic And Covalent Bonds Gizmo : Ionic Bonds Gizmo Worksheet Answers - worksheet : The main difference between ionic bonds and covalent bonds is sharing of electron pairs and atoms.. The ionic bonds gizmo™ allows you to explore how ionic bonds form. For instance, positively charged sodium ions and negatively charged chloride ions bond together to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge. They can be easily broken into its primary structure as. Each bond consists of a shared pair of electrons, and is very strong. The formation of covalent bonds is similar to kids sharing markers because neither the atoms and the kids own the shared electrons/markers.
You can calculate the charge of an atom by subtracting the. The primary difference between covalent and ionic bonding is that with covalent bonding, we must invoke quantum mechanics. For instance, positively charged sodium ions and negatively charged chloride ions bond together to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge. The two main types of chemical bonds are ionic and covalent bonds. Simple molecular substances and giant covalent structures have different properties.

Add to collection(s) add to saved.
Unlike ionic binding, electrons are shared between both atoms in covalent bonds, so the electrons spend time around. Get free answer key to ionic bonds gizmo. Select a metal and a nonmetal atom, and transfer electrons from one to the other. Ionic and covalent bonds are the two extremes of bonding. Covalent bonds are usually found in atoms that have at least two, and usually less than seven, electrons in their outermost energy shell, but this is not a hard and fast rule. I concluded that sodium chloride and sodium bicarbonate contained ionic bonds because they both conducted electricity and were solid at room temperature. The ionic bonds gizmo™ allows you to explore how. In a covalent bond, the atoms bond by sharing electrons. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. For instance, positively charged sodium ions and negatively charged chloride ions bond together to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge. Simulate ionic bonds between a variety of metals and nonmetals. As mentioned above, ionic bonds are a result of electrostatic forces between atoms that get attracted towards each other due to the possession of opposite electrical charges. This kind of bond generally involves nonmetals.
The ionic bonds gizmo™ allows you to explore how ionic bonds form. Wont need as much heat to melt. About covalent and ionic bonds. Covalent bonds are usually found in atoms that have at least two, and usually less than seven, electrons in their outermost energy shell, but this is not a hard and fast rule. A covalent bond is a bond that results from the sharing of pairs of electrons between two atoms.

Ionic and covalent bonds are the two extremes of bonding.
The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the exploring ionic and covalent bonds gizmo : The formation of covalent bonds is similar to kids sharing markers because neither the atoms and the kids own the shared electrons/markers. An ionic bond is a chemical bond between two dissimilar (i.e. The main difference between ionic bonds and covalent bonds is sharing of electron pairs and atoms. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The primary difference between covalent and ionic bonding is that with covalent bonding, we must invoke quantum mechanics. Simple molecular substances and giant covalent structures have different properties. In contrast to metallic bonding neither ionic nor covalent bonding form free electrons, therefore ceramic materials have very low electric conductivity and thermal conductivity. Covalent bonds usually occur between ionic and covalent compounds also differ in what happens when they are placed in water, a exploring our fluid earth, a product of the curriculum research & development group (crdg). The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when covalent bonds have a definite and predictable shape and have low melting and boiling points. Use simulation to observe properties of ionic and molecular compounds in conjunction with msds sheets. Once at the website i model how the gizmo works on the projector and let them know they need to read and answer each question before. Ionic and covalent bonds are the two extremes of bonding.
Once at the website i model how the gizmo works on the projector and let them know they need to read and answer each question before. Ionic and covalent bonds are the two extremes of bonding. Covalent bonds are usually found in atoms that have at least two, and usually less than seven, electrons in their outermost energy shell, but this is not a hard and fast rule. In a covalent bond, the atoms bond by sharing electrons. Add to collection(s) add to saved.
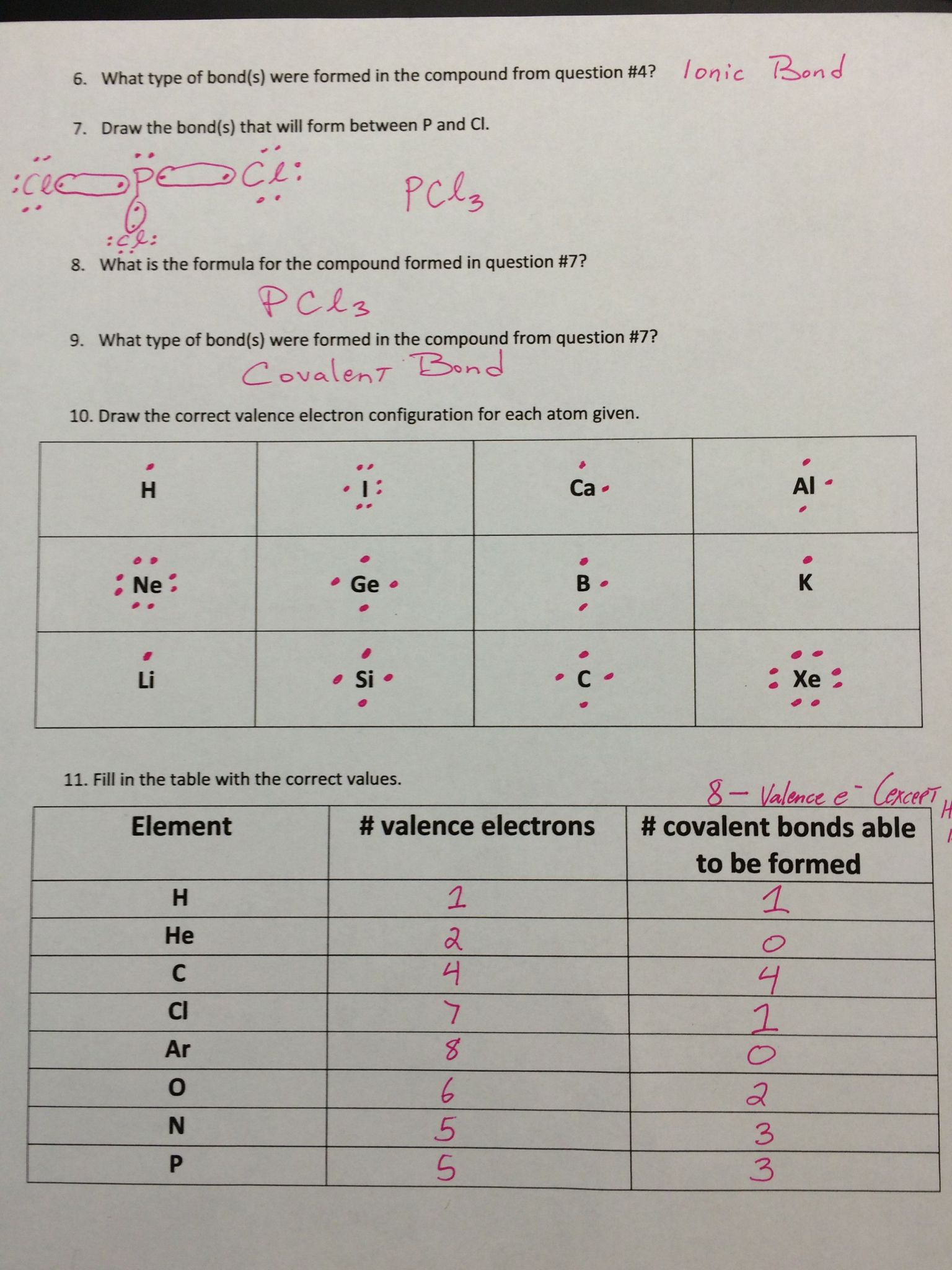
The ionic bonds gizmo™ allows you to explore how.
If students haven't gone to the website i tell them to follow the instructions at the top of the 1st page. Download mobi ionic bonds student exploration gizmo answer key book pdf free download link or read online here in pdf. Explorelearning launch gizmo choose a substance, and then move electrons between atoms to form covalent bonds and build molecules. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between atoms in a covalent bond. The primary difference between covalent and ionic bonding is that with covalent bonding, we must invoke quantum mechanics. Most metals can conduct and ionic has metal its bond. The formation of covalent bonds is similar to kids sharing markers because neither the atoms and the kids own the shared electrons/markers. Covalent bonds usually occur between ionic and covalent compounds also differ in what happens when they are placed in water, a exploring our fluid earth, a product of the curriculum research & development group (crdg). To begin, check that sodium (na) and chlorine (cl) are selected from the menus at form a bond: An ionic bond is a chemical bond between two dissimilar (i.e. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when covalent bonds have a definite and predictable shape and have low melting and boiling points. The ionic bonds gizmo™ allows you to explore how ionic bonds form. Covalent bonds and ionic bonds are two different ways of how elements bond to each other.
Komentar
Posting Komentar